does saliva kill hiv
 Myths about HIV and AIDS | Avert
Myths about HIV and AIDS | AvertArticle Contents Oral Transmission of Human Immunodeficiency Virus by Infected Seminal Fluid and Milk: A Novel Mechanism Cite amuel Baron, Joyce Poast, C. Joan Richardson, Derrick Nguyen, Miles Cloyd, Oral Transmission of Human Immunodeficiency Virus by Infected Seminal Fluid and Milk: A Novel Mechanism, The Journal of Infectious Diseases, Volume 181, Issue 2, February 2000, Pages 498–504, Abstract It was proved whether seminal fluid and milk successfully transmit HIV orally overcoming the salivary hypothonic inactivation of the HIV transmitters receptor. Isotonic salt solution and normal samples of milk donators, collostrum, seminal fluid and blood were studied for their ability to overcome saliva hypothonic inactivation. All samples, in physiological volumes, prevented the saliva hypotony from inactivating leukocytes that transferred HIV by providing solutes and delayed diffusion. This indicates that the successful oral transmission of HIV by seminal fluid, milk and colostrum can be due to its isotonic, which exceeds the hypotony salivary inactivation of the leucocytes that transmit HIV. The oral transmission of the human immunodeficiency virus (HIV) by the 30 million HIV carriers is rare during the kiss, bite, aerosolization and dental treatment, even when the infected or exudated blood is spilled in the mouth []. This phenomenon is reportedly due to salivary inactivation of HIV-infected leukocytes that are shed into the mouth []. The interruption of the leukocytes of Saliva has been confirmed in recent studies []. Leucocyte inactivation is attributable primarily to the strong hypothonic saliva, although other less active HIV inhibitors are also present in saliva [, ]. However, oral transmission of HIV occurs epidemiologically if the infected seminal fluid [] or the milk [] is deposited orally, despite the presence of saliva. By using patient samples, we study the mechanisms by which the seminal fluid or the carrier's milk can overcome the protection by the in vitro saliva of the receptor. Materials and MethodsSpecimenes Six samples of normal donor milk were collected 3-4 days after delivery. Three samples of normal donor collostrum were collected 0–2 days after delivery. Saliva samples were collected from 6 regular donors and centrifuged and filtered through a 0.22-μm filter for sterilization. Seminal fluids were collected from 3 normal men. The samples were stored at 4°C for 1–2 days or, alternatively, stored at −20° C for up to 3 months. This storage was determined not to affect the action of seminal liquid, milk or collostrum. Blood was obtained from 2 regular donors and was used immediately. VirusSchemas of HIV strain 213 spread into human H9 lymphocytes through standard procedures []. A second strain of HIV, AC-1, is equally well inhibited by mononuclear leukocytes of saliva blood treated with peripheral blood (PBL) []. Aliens were stored frozen at −70°C. The Indiana strain of the vesicular stomatitis virus (VSV) was propagated in L murine cells, as described in other places []. The VSV stocks were frozen at −70°C.CellsThe Human PBL and the CEM human lymphocyte cell line were prepared and propagated at RPMI 1640 medium with 10% fetal serum and bovine antibiotics, as described elsewhere []. The macrophogenes of human peripheral blood were obtained by normal PBL ficoll-hipaque purification []. To obtain macrophages, the purified PBL was allowed to adhere to a glass surface for 3 h to 37°C. Non-attacked mononuclear leukocytes are rinsed with medium, and adhering macrophages were used in cell feasibility experiments in the presence of saliva. Virus Multiplication The effects of the various treatments on virus multiplication were determined in human CEM lymphocytes infected by viruses, PBL or L929 cells. HIV multiplication in the PBL was determined as the performance of 2 × 106 ficoll-hipaque-purified normal PBL that had been cultivated with phyohemagglutimn (4 μg/mL) for 2 days before treatment with 20 U/mL interleukin (IL)-2 (40 U/mL) for 2 additional days and then infected with 105 TCID50 ce Infected cells were incubated with 20 U/mL IL-2 for 5 days, washed 4 times, and incubated with saliva without diluting, the various mixtures, or cultural media for 15–60 min. These leukocytes were subsequently washed and cultivated for the production of HIV in RPMI 1640 tissue medium plus 15% bovine fetal serum and 20 U/mL IL-2 for 24 h before the cell-free medium was harvested for the HIV performance test, as described below. In experiments that used the human CEM lymphocyte cell line [], multiplication was determined as the performance of HIV infectious from 6 × 105 CEM lymphocytes infected with 105 TCID50 of the HIV strain 213. The cells were incubated for 4 days and washed 4 times before treatment for 15 to 60 minutes with the different preparations. These infected lymphocytes were then washed 4 times and cultivated for 24 hours before being harvested for testing HIV production, as described below. For experiments that require non-maintenance facilities in which to perform more complex procedures, we replace a safer surrogation virus, vesicular stomatitis virus (VSV), instead of HIV. Other substitute viruses have been used previously []. The VSV multiplication was measured, similarly, as the performance of the infectious virus in the cultural environment of CEM lymphocytes or murine L cells []. This was done after infection with 3000 pfu virus. Pilot experiments were initially performed with human CEM lymphocytes infected by VSV or L murine cells. Confirmational experiments were performed with HIV-infected CEM lymphocytes []. All studies were replicated 3 times. HIV and VSV production testsThe production of HIV in the experiments was determined by the standard TCID50 test with human lymphocytes MT-2 []. Specifically, the cultural fluids harvested from the human CEM lymphocytes infected with HIV [] were diluted in increases of 0.5-log10 in the RPMI culture medium containing a fetal bovine serum. Next, 50 μL of each dilution was added to quadrupled microtiter wells, each with 110 μL of 2 × 104 MT-2 cells in the middle of cultivation. After the series dilutions, 120 μL of a nutrient was added to each well. These microtiter plates were incubated at 37° C for 3 days in a CO2 incubator and then were fed again by a 10% bovine fetal serum. The wells were read for the cytopatic effect of multinucleated giant HIV cells on day 5 or 6. The TCID50 was determined by the method of Reed and Muench [], and the production of VSV was determined as pfu in L cells []. Statistics In addition to the basic descriptive statistics in the legends of the figure, statistical methods such as regression analysis and non-parametric methods were used, without adjustments for multiple tests. In each case, the relevant trends and differences observed were large, and all related significance levels (P values) were small, even with sample sizes used. ResultsReversal of Salivary Inactivation of Virus-Infected Leukocytes by Raising the Tonicity of Saliva with Seminal Fluid, Milk, or Isotonic Salt Solution We hypothesize that the restitution of salts in hypothonic saliva by isotonic seminal fluid deposited orally, milk or colostrum would reverse the ability of salcreiva to inactivate the infected viruses. To determine the proportion of saliva to an isotonic salt solution that could prevent cellular lysis, we diluted in saliva series in variable increases in the balanced isotonic salt solution of Dulbecco. The experiments were performed first with the sub-restricted VSV (see Methods) and then with HIV. In the first experiments, dilutions were used to treat VSV-infected L929 cells for 1 h to allow cell inactivation. The treated cells were washed and reused in the middle for 24 h incubation for the production of viruses, as described above. Because the virus can only occur by surviving viable cells, any decrease in virus performance would indicate the inactivation of saliva-infected cells []. As shown in , the strong inhibition of cell virus production Infected L occurred with saliva mixtures and isotonic balanced salt solution containing saliva 70%–90%, thus confirming that infected cells were inactivated by high saliva concentrations and therefore did not produce viruses. The reduced inhibition of the virus's performance occurred with the mixtures containing saliva from 35% to 65%, showing partial inactivation of the cells by saliva and thus indicating partial protection of the cells. Significant inhibition of virus production occurred with A, Salivar inhibition reversal of the vesicular stomatitis virus (VSV) multiplication in infected L cells. Saliva was diluted in isotonic balanced salt solution in increments shown. Each diluted sample was incubated for 1 h with L929 murine cells infected by VSV in 4 replicated microtiter wells. The cells were washed 3 times with Hanks' balanced salt solution, nourished Eagle's basal medium that contained the fetal serum of the fetal bovine, and incubated for 18 h before the supernatants were harvested to determine the production of viruses, using the test of virus plaque in L929 cells. The Log10 performance inhibition VSV was calculated by comparing the virus's performance in the control cultures with that of the treated cultures. B, Salivar inhibition of VSV multiplication in CEM lymphocytes by dilution in medium human seminal liquid or milk. The procedures were like A except that the saliva was diluted in seminal fluid, milk, colostrum or medium, and 6 × 105 human CEM lymphocytes infected by VSV, instead of L929 cells, were treated with samples. C and D, Seminal fluid or milk dilution, respectively, prevents the inhibition of the saliva of the human immunodeficiency virus (HIV) multiplication in CEM lymphocytes. The procedures were as in B, except that the CEM lymphocytes infected with HIV were treated with saliva samples diluted in seminal fluid or milk, respectively. E, Blood dilution prevents saliva inhibition of HIV multiplication in normal human peripheral blood cells (PBL). The procedures were like in C except that the blood was used instead of milk, and the PBL was replaced with CEM cells. F, Blood, Milk, Collostrum, and Seminal Fluid prevents the inhibition of the saliva of HIV multiplication in the human PBL. The procedures were like C-E. *P A, Reversal of salivaryhibition of vesicular stomatitis virus (VSV) multiplication in infected L cells. Saliva was diluted in isotonic balanced salt solution in increments shown. Each diluted sample was incubated for 1 h with L929 murine cells infected by VSV in 4 replicated microtiter wells. The cells were washed 3 times with Hanks' balanced salt solution, nourished Eagle's basal medium that contained the fetal serum of the fetal bovine, and incubated for 18 h before the supernatants were harvested to determine the production of viruses, using the test of virus plaque in L929 cells. The Log10 performance inhibition VSV was calculated by comparing the virus's performance in the control cultures with that of the treated cultures. B, Salivar inhibition of VSV multiplication in CEM lymphocytes by dilution in medium human seminal liquid or milk. The procedures were like A except that the saliva was diluted in seminal fluid, milk, colostrum or medium, and 6 × 105 human CEM lymphocytes infected by VSV, instead of L929 cells, were treated with samples. C and D, Seminal fluid or milk dilution, respectively, prevents the inhibition of the saliva of the human immunodeficiency virus (HIV) multiplication in CEM lymphocytes. The procedures were as in B, except that the CEM lymphocytes infected with HIV were treated with saliva samples diluted in seminal fluid or milk, respectively. E, Blood dilution prevents saliva inhibition of HIV multiplication in normal human peripheral blood cells (PBL). The procedures were like in C except that the blood was used instead of milk, and the PBL was replaced with CEM cells. F, Blood, Milk, Collostrum, and Seminal Fluid prevents the inhibition of the saliva of HIV multiplication in the human PBL. The procedures were like C-E. *P Another possibility is that enough bleeding in the mouth can contribute enough to the tonic to overcome the salivary inactivation of HIV-infected leukocytes in the shed blood []. Accordingly, we determine whether the dilution of saliva in heparinized blood prevented the inhibition of HIV-positive HIV multiplication in the HIV-infected human PBL. As shown in , blood dilution prevented saliva inhibition from HIV multiplication, with a dilution response curve similar to those obtained through the use of seminal salt, milk, colostrum and isotonic solutions. The controls did not show HIV inhibition in the heparin concentration used. Thus, heavy bleeding in the mouth may pose a danger to health workers and other contacts. There is a HIV transmission case report dying during heavy bleeding in the mouth that supports this possibility []. To confirm the findings obtained with the cell line of CEM lymphocytes that the hypothonic saliva dilution with isotonic body fluids exceeds the protective action of the saliva, we repeat the experiments with normal human PBL. The findings confirm for the HIV-infected PBL those obtained through the use of CEM lymphocytes (). Below we calculate whether the volumes of seminal liquid deposited orally, milk, collostrum or blood were sufficient to overcome saliva protection. Because the residual volume of saliva in the mouth averages 0.75 mL [], we can estimate that the volume of seminal fluid deposited orally, milk or collostrum required to partially protect your infected leukocytes against the salivar lysis is 0.25–0.45 mL. The full protection of the shed leukocytes is expected to occur with volumes deposited ю0.45 mL (50 mL of milk deposited or collostrum. The blood above 0.45 mL would also prevent salivar inactivation. Therefore, the usual volumes of seminal fluid, milk or collostrum deposited in the mouth are sufficient to overcome the normal protection of the saliva of the HIV-infected leukocytes and, therefore, can account for the successful oral transmission by these fluids. The penetration of fluids and physical milks prevented by the Hypotonicity of Saliva? Effect of incubation time with seminal liquid or milk on salivary inactivation of leucocytes infected by virus To help determine whether the seminal fluid and leucocytes protected by milk infected with the surrogate VSV, not only with its tonicity but also by physical diffusion of the solutes and water, we determine the time necessary for the hypothonic to impregnate the infected milk. Saliva (450 μL) was layered more than 50 μL of seminal fluid, milk, or colostrum containing 2 × 106 CEM lymphocytes and was then incubated for 15 or 60 minutes before being tested for the inactivation of infected leucocytes. As shown for milk in , the longer incubation time of 60 minutes increased the inhibition of the virus's performance, indicating a decrease in the protection of cells infected by the virus by longer contact with saliva. Similar results were obtained with seminal fluid. These findings indicate that seminal fluid and milk can delay the penetration of salivar water and the external diffusion of the fluid and seminal milk solutes, protecting partly their virus-infected leukocytes. Human milk protects CEM lymphocytes infected by the vesicular stomatitis virus (VSV) exposed to saliva: effect of incubation time. The procedures were as in except that 450 μL of saliva was escaped (without mixing) more than 50 μL of milk containing 5 × 106 CEM lymphocytes infected and was incubated for 15 or 60 min. * P Human milk protects the virus from vesicular stomatitis (VSV) infected with CEM lymphocytes exposed to saliva: effect of incubation time. The procedures were as in except that 450 μL of saliva was escaped (without mixing) more than 50 μL of milk containing 5 × 106 CEM lymphocytes infected and was incubated for 15 or 60 min. * Effect of the volume of seminal fluid or milk in salivary inactivation of virus-infected leucocytes. Another diffusion indicator is the effect of the size of the drop of seminal fluid and milk in the ability of the saliva to inactivate infected leucocytes. We hope that the leukocytes contained in larger drops of seminal liquid or milk will be more resistant to inactivation, because their larger radio would delay the diffusion of the solutes and water. To test this possibility, we have stratified the saliva on different volumes of seminal liquid or milk keeping the saliva-a-show ratio at 10 : 1. In the representative experiment shown in , greater is the volume of colostrum, less the inactivation of human CEM lymphocytes infected by VSV by saliva. Findings suggest that larger drops of seminal fluid, milk or collostrum could provide greater protection of lymphocytes infected by the virus, indicating a reduction in the spread between saliva and these fluids. Collostrum protects CEM lymphocytes infected by the vesicular stematitis virus (VSV) against saliva inactivation: effect of the volume of collostrum. The procedures were as in , with 60 min incubation, except that the volumes of colostrum containing infected CEM lymphocytes were 0.025, 0.1, and 0.5 ml and saliva volume was properly increased to maintain 10 : 1 saliva-a-muestra ratio. * P Colostrum protects CEM lymphocytes infected by the vesicular stomatitis virus (VSV) against saliva inactivation: effect of the volume of collostrum. The procedures were as in , with 60 min incubation, except that the volumes of colostrum containing infected CEM lymphocytes were 0.025, 0.1, and 0.5 ml and saliva volume was properly increased to maintain 10 : 1 saliva-a-muestra ratio. * Effect of saliva mixing with seminal liquid or milk in the inactivation of leucocytes infected by viruses If seminal fluid and milk slow the diffusion of the solutes and salivar water, the mixture should improve the diffusion. To test that possibility, we compare layers or mixtures of saliva and samples, with a ratio of 9 : 1 (0.9 mL of saliva plus 0.1 mL of milk containing 2 × 106 CEM lymphocytes). In the representative experiment shown in , mix, compared to the saliva layer with milk, greater inhibition of the returns of the virus. The results indicate that the mechanical mixture of saliva milk can decrease the inactivation of leucocytes infected by the milk virus, presumably promoting the diffusion of solutes and water. Human milk protects CEM lymphocytes infected by the vesicular stomatitis virus (VSV) exposed to saliva: mixing effect. The procedures were as in except that 0.9 ml of saliva was or was dragged or mixed with 100 μL of CEM lymphocytes infected in the milk. * Human milk protects the CEM lymphocytes infected by the vesicular stematitis virus (VSV) exposed to saliva: mixing effect. The procedures were as in except that 0.9 ml of saliva was or was dragged or mixed with 100 μL of CEM lymphocytes infected in the milk. * Effect of saliva on HIV without cellsIn comparison to the inactivation of leucocytes infected with saliva, we study the ability of saliva to inactivate any HIV without cells that could occur in saliva. Previous reports showed a 3 to 5-fold inhibition of saliva-cell-free HIV [], compared to the inhibition of 10,000 infected cells []. In our experiments, saliva incubation at 37°C with HIV without cells for 2 h resulted in an average 3-fold inhibition. This finding agrees with previous reports and with the only moderate inactivation of HIV free by hypothonic fluids such as water []. Therefore, saliva seems to inactivate HIV free cells with less force than HIV-infected leukocytes. Effect of saliva on the viability of macrophages Our studies have used the line of human CMS or normal PBMC lymphocytes that are mainly lymphocytes []. However, HIV can also be transmitted by infected macrophages []. Therefore, we determine if normal human macrophages could be killed by saliva. The mononuclear leukocytes purified by the hypola adherence to a glass surface for 3 h and then were treated with saliva or medium of control not diluted for 15 min. The cultures were washed and stained with the Wright stain and were observed for morphological disturbance []. The macrophages treated with saliva were interrupted .90%, as was previously found for lymphocytes and, more recently, also for macrophages [, ]. This finding indicates that macrophages and lymphocytes are inactivated by saliva. Discussion It has been reported that the rarity of the salivary transmission of HIV is mainly due to the salivary inactivation of leucocytes who transmit HIV by the strong hypothetical of saliva []. Saliva may inhibit the production of HIV leukocytes by 10,000 in vitro, compared to the inhibition of three to five times reported HIV-free cells for salivary inhibitors [, ]. This conclusion on cell transmission is supported by the following evidence: most HIV infectivity in body fluids and secretions of carriers is found in infected leucocytes instead of as a free infectious virus []; infected leucocytes can directly infect epithelial cells in mucous surfaces []; any cellless infectious HIV observations in mucous surfaces Compared to saliva, other mucous secretions (e.g. vaginal) are isotonic; they maintain the viability of HIV-infected leukocytes and are present in transmission sites []. Previously unexplained is that oral transmission of HIV occurs epidemiologically if it is deposited orally seminal fluid infected [] or milk [], despite the presence of saliva. We hypothesize that the liquid and the isotonic milk deposited orally could protect your HIV-infected leukocytes by reconstituting saliva tonicity or physically excluding saliva hypotony. We found that the restitution of tonicity by adding seminal fluid, milk, collostrum or balanced salt solution prevented the salivar inactivation of mononuclear leukocytes infected by the virus. In addition, the physiological volumes of seminal fluid, milk and collostrum deposited in the mouth are sufficient to protect leukocytes against saliva and allow the transmission of HIV. These findings are consistent with the hypothesis that the epidemiological transmission of HIV orally by seminal fluid, milk and colostrum may be due to its isotonic, which prevents the saliva inactivation of the transmitters' hypotony. The restitution of tonicity can be regulated by additional mechanisms. Because mucous secretions are more viscous than saline solution, they can physically kidnap their leukocytes and thus delay salivar inactivation by preventing the diffusion of hypothonics. A mechanism for sequestration of protection would predict that the protection of leukocytes by seminal liquid and milk would be overcome by a longer incubation time, smaller droplets and mix. We find these predictions that should occur, indicating that the salivary mechanisms of protection against HIV-infected leukocytes in seminal fluids deposited orally, milk and collostrum are due both to the reconstitution of tonicity and the abduction of infected leukocytes. Thus, oral cavity is unique among the mucous surfaces to have a defensive oral barrier to HIV transmission and also probably to the human T cell leukemia virus [, ]. This main defense for the salivary inactivation of the transmitter leukocytes, with the additional help of other salivary inhibitors [], seems to explain the rarity of the oral transmission of HIV. The oral barrier can be overcome, however, by seminal fluid, milk and collostrum, thus accounting for the epidemiological transmission observed by these mucous secretions [].Also medically important is that the salivary defense has directed the attention to the most likely mucousal transmitter, the HIV-infected leucocyte, more than the least likely HIV. If the right goal to prevent mucosa transmission is the HIV-infected leucocyte, then anticellular solutions that are even more powerful than hypothonic could be applied topically in other mucus sites (e.g. vagina and rectum). In these other sites, the rate of vaginal transmission of HIV by seminal fluid is 0 % per year of exposure, while the risk of transmission of HIV to newborn is 0.5% []. We are currently studying a number of topical anticellular substances other than those reported to be non-oxinol-9 ineffective. These preventive candidates include disinfectants for blood products, bile detergents and commercial vaginal products, which are naturally produced or used. Acknowledgements We acknowledge the valuable statistical analysis of Elbert Whorton; we thank Rhonda Peake for the outstanding editorial organization. References Presented in part: Third National AIDS Malignancy Conference, Bethesda, Maryland, 26–27 May 1999. The approval guidelines of the institutional review board, including informed consent, of the medical branch of the University of Texas were followed in clinical research. Email Alerts More about this topic Related Articles in Related Articles in PubMedCiting ConnectResources ExploreOxford University Press is a department at Oxford University. In addition, the University's goal of excellence in research, scholarship and education by publishing around the world or This PDF is available for Subscribers OnlyFor full access to this pdf, enter an existing account or purchase an annual subscription.
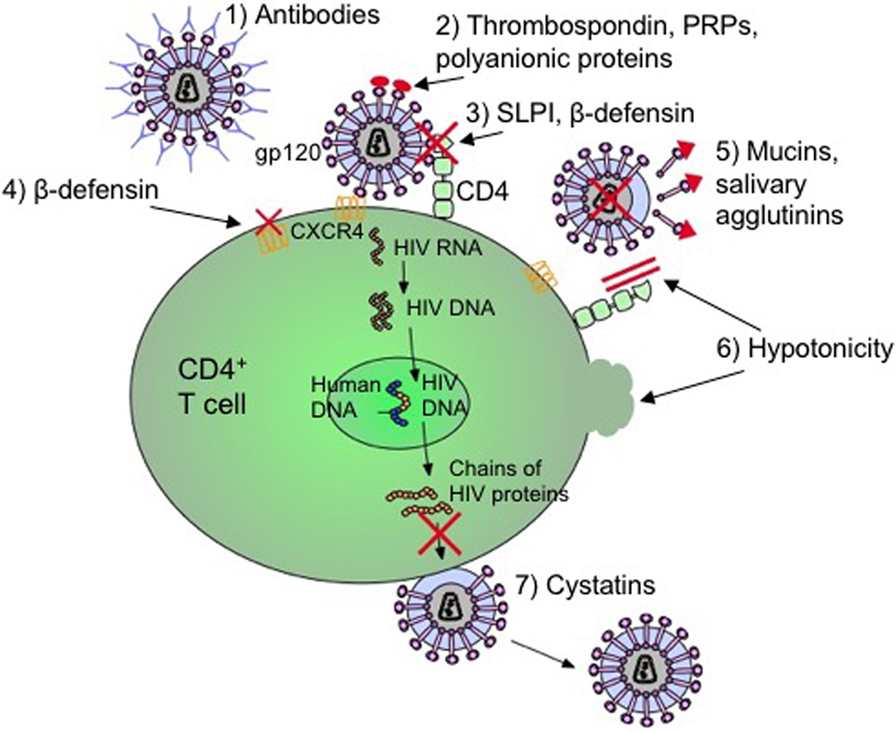
Frontiers | HIV Infection and Compromised Mucosal Immunity: Oral Manifestations and Systemic Inflammation | Immunology
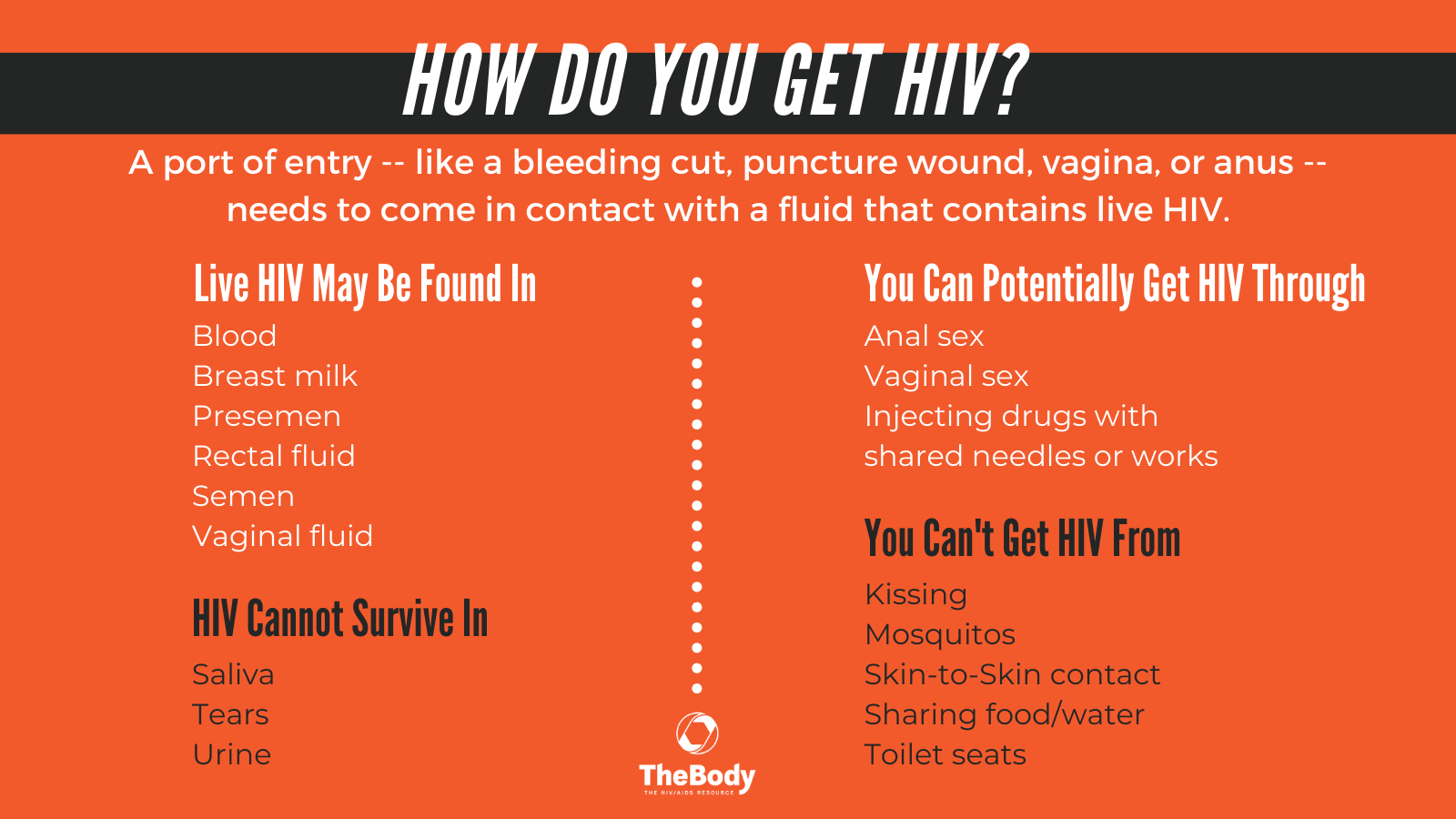
HIV/AIDS: Signs, Symptoms, Causes, Treatments, and much more.
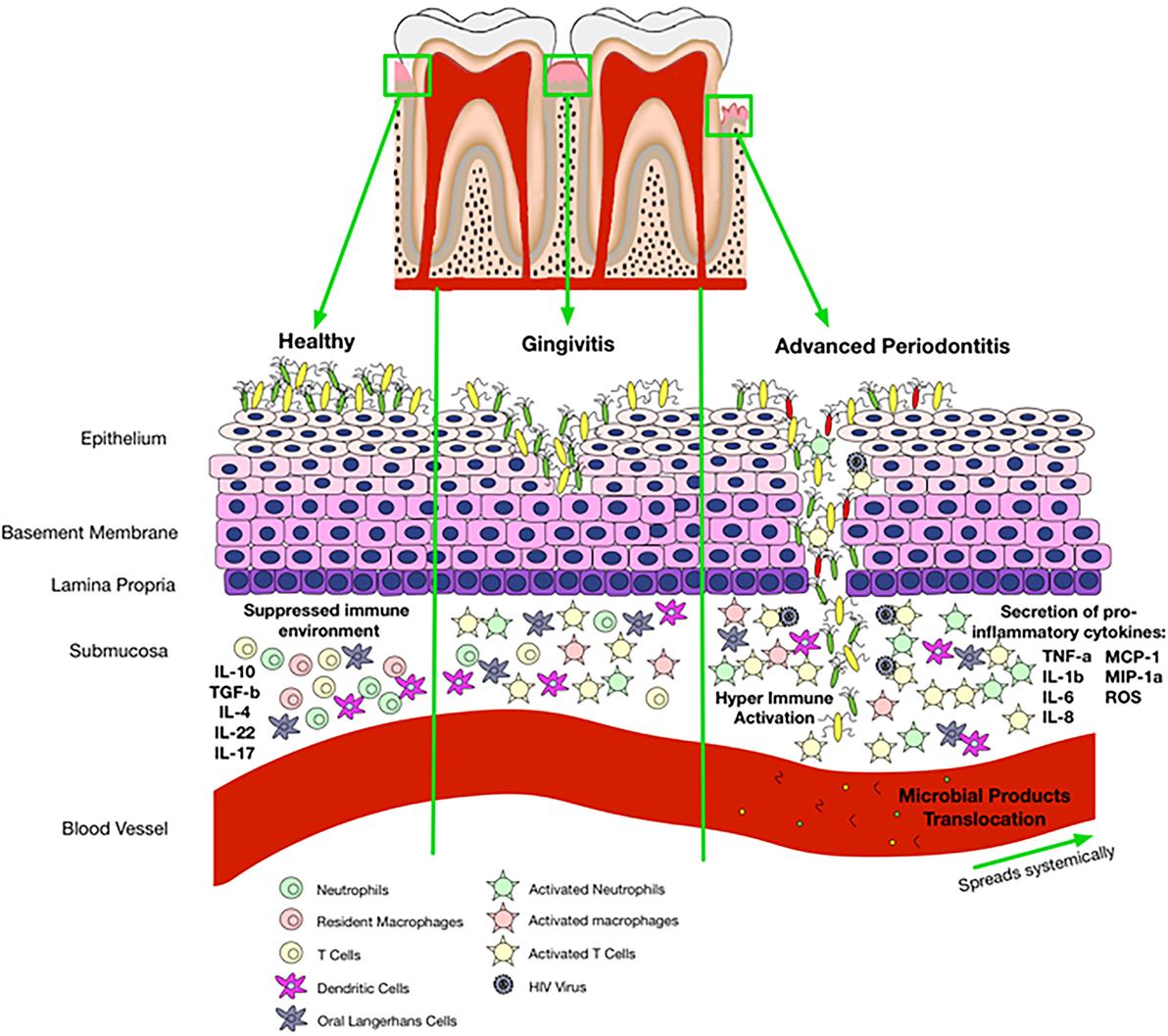
Frontiers | HIV Infection and Compromised Mucosal Immunity: Oral Manifestations and Systemic Inflammation | Immunology

Myths about HIV and AIDS | Avert

The Virus - The Virus | The Age Of Aids | FRONTLINE | PBS

Impossible routes of HIV transmission | aidsmap

Human Immunodeficiency Virus (HIV) Infection - Infections - MSD Manual Consumer Version

Oral Sex and HIV: Likelihood, Risks, Prevention, and More

How is HIV transmitted?

Is HIV Transmitted Through Kissing? How HIV Is Transmitted
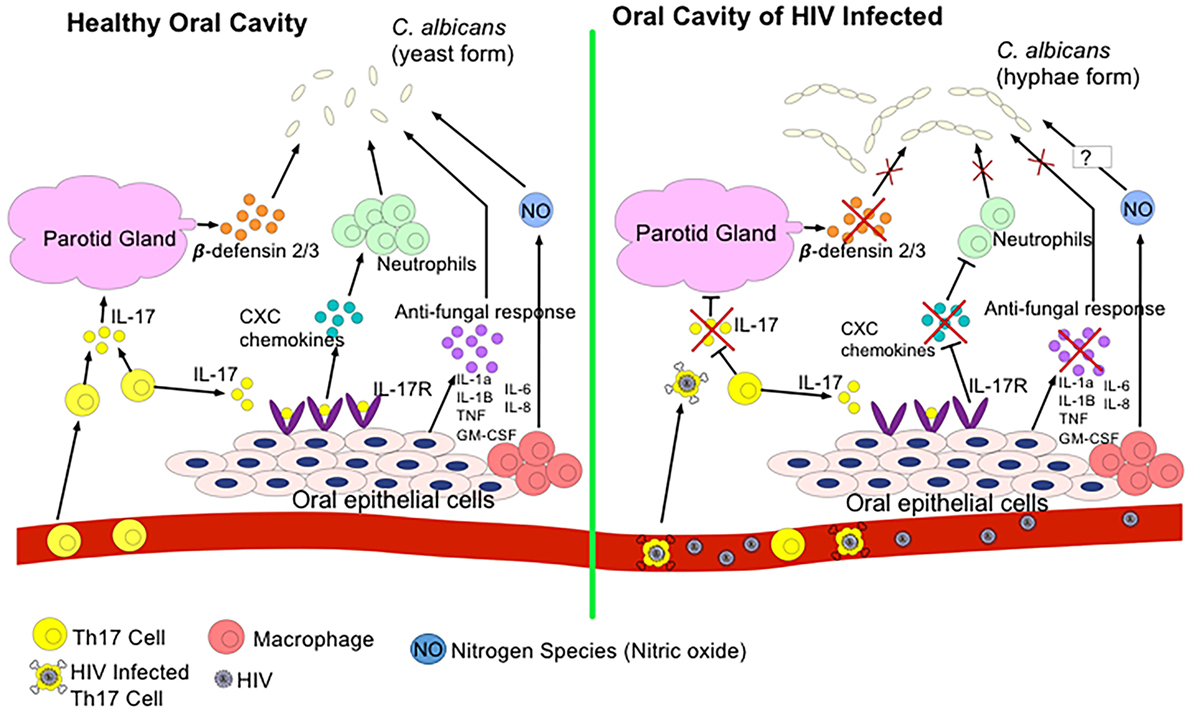
Frontiers | HIV Infection and Compromised Mucosal Immunity: Oral Manifestations and Systemic Inflammation | Immunology

Human Immunodeficiency Virus (HIV) Infection - Infectious Diseases - MSD Manual Professional Edition

Saliva Does Not Transmit HIV - POZ

Myths about HIV and AIDS | Avert
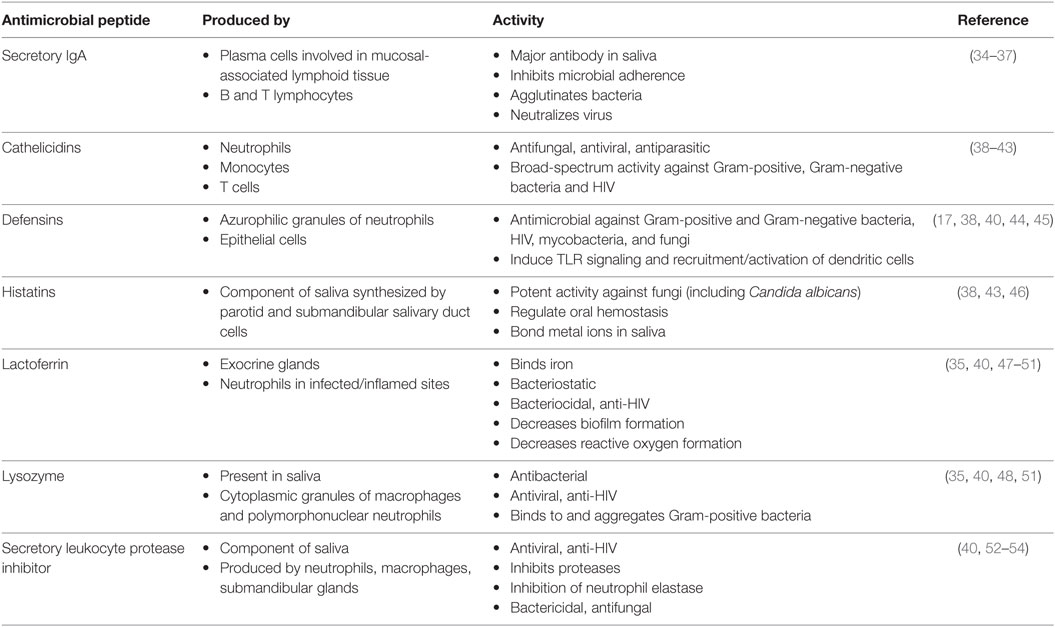
Frontiers | HIV Infection and Compromised Mucosal Immunity: Oral Manifestations and Systemic Inflammation | Immunology
HIV and AIDS Tutorial

Why Can't Mosquitoes Transmit HIV? | Viruses101 | Learn Science at Scitable

What is HIV/AIDS? (article) | HIV and AIDS | Khan Academy

HIV 1: epidemiology, pathophysiology and transmission | Nursing Times

Is it possible for a mosquito to spread HIV/AIDS? - Quora

Myths about HIV and AIDS: Transmission and misconceptions
/GettyImages-590279757-9b886dbd225a485fb8d2e1a2c09ca484.jpg)
The Meaning of a Positive HIV Test

What Do You Really Know About HIV and AIDS?

HIV 1: epidemiology, pathophysiology and transmission | Nursing Times

Why Can't Mosquitoes Transmit HIV? | Viruses101 | Learn Science at Scitable

Kaposi's sarcoma and HIV | aidsmap
The tea on HIV | WHO | Regional Office for Africa
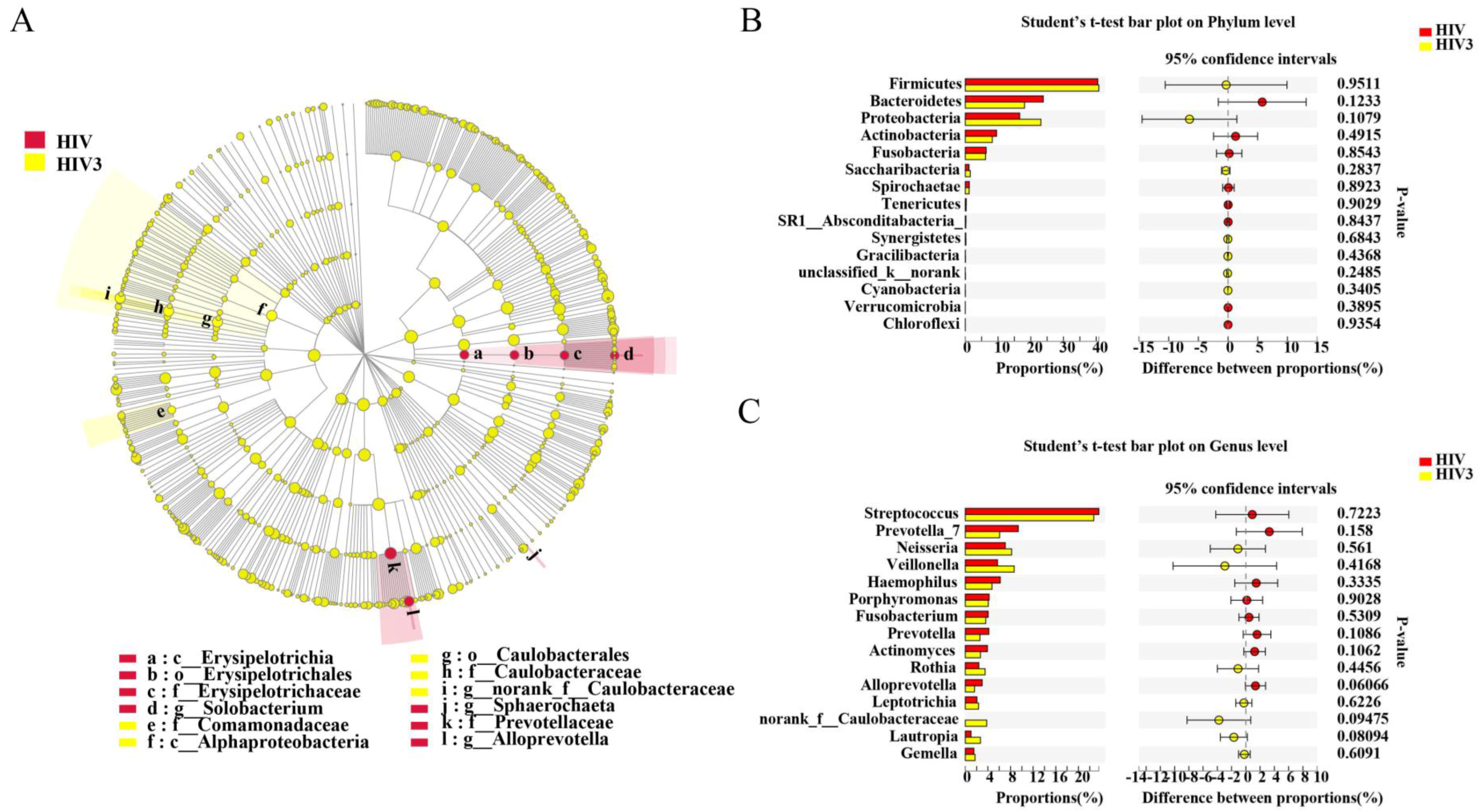
Pathogens | Free Full-Text | Altered Salivary Microbiome in the Early Stage of HIV Infections among Young Chinese Men Who Have Sex with Men (MSM)

HIV Transmission and Risks - POZ

Countdown to a Cure: The Effort to Finally Defeat AIDS | UC San Francisco

HIV/AIDS - Wikipedia
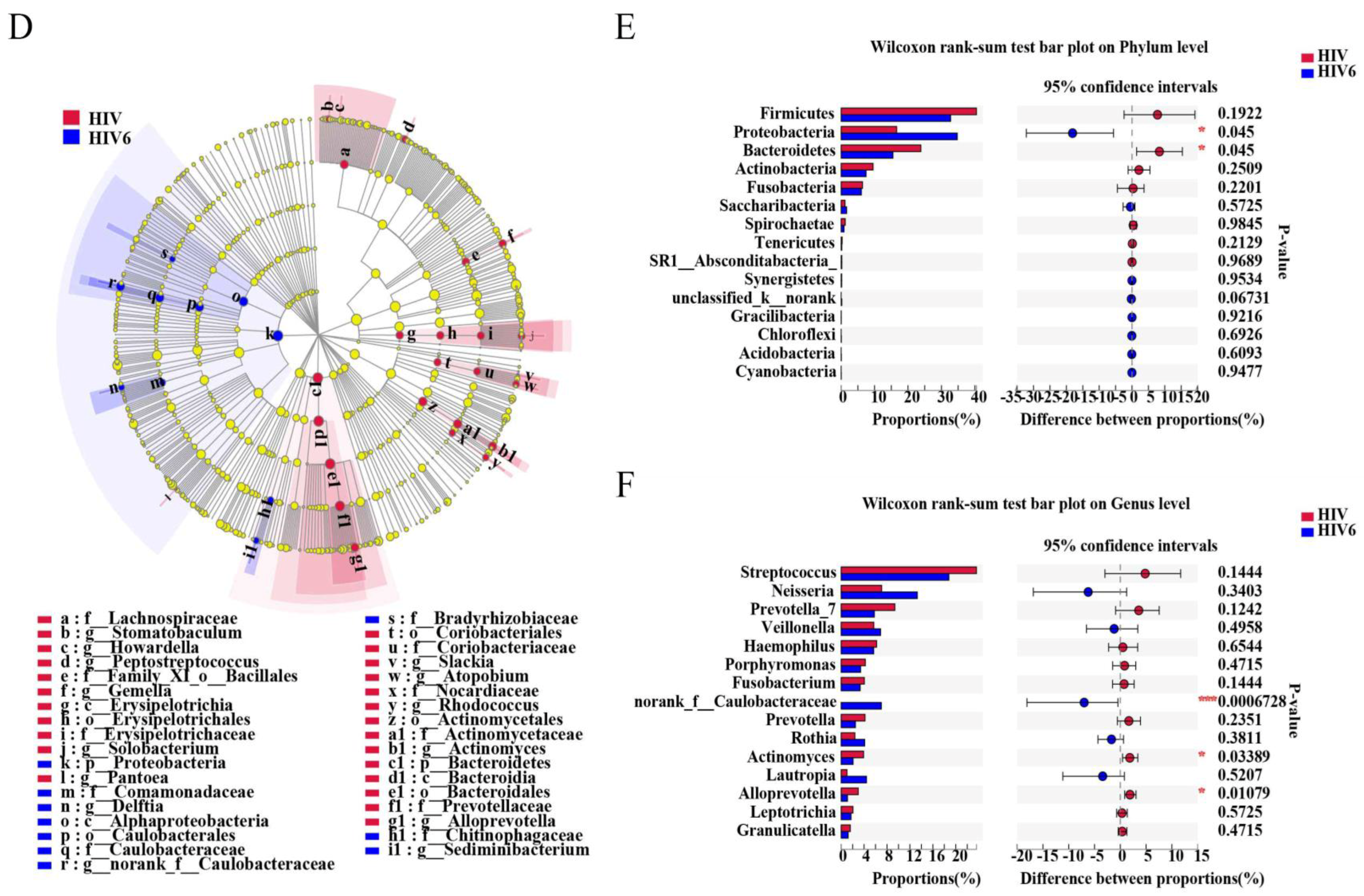
Pathogens | Free Full-Text | Altered Salivary Microbiome in the Early Stage of HIV Infections among Young Chinese Men Who Have Sex with Men (MSM)
/hiv-aids-symptoms-4014373-final-hl-4413b4d0c85b4b99b73431a97beb5224.jpg)
HIV Infection: Signs, Symptoms, and Complications
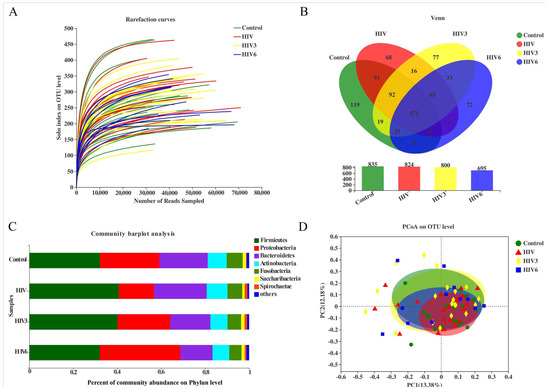
Pathogens | Free Full-Text | Altered Salivary Microbiome in the Early Stage of HIV Infections among Young Chinese Men Who Have Sex with Men (MSM)
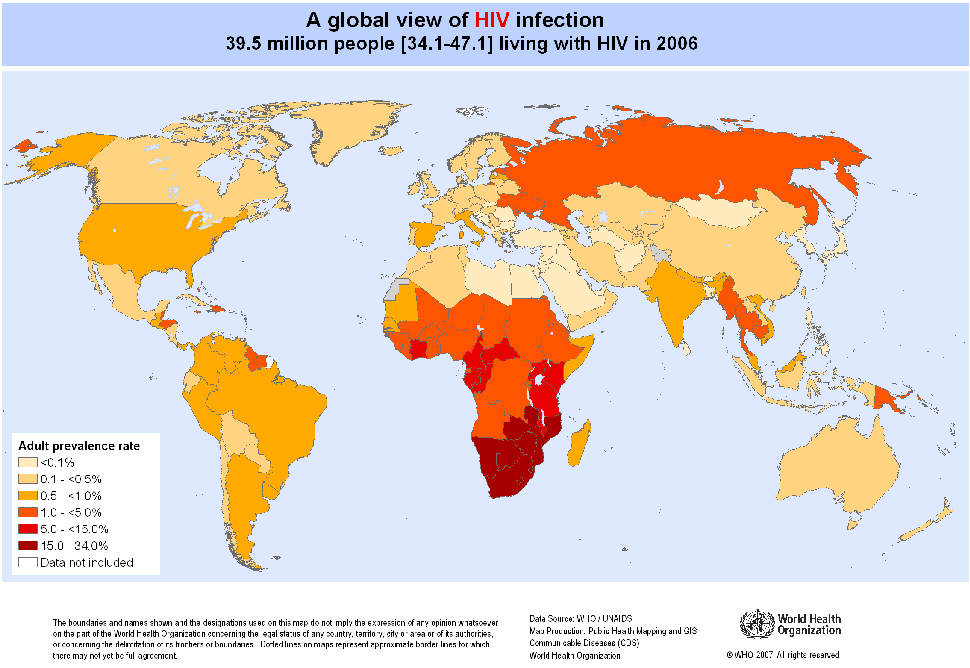
HIV Infection and AIDS

HIV Transmission | The Well Project
A Visual Guide to HIV/AIDS
What Are HIV and AIDS? | HIV.gov

HIV Transmission and Risks - POZ
Posting Komentar untuk "does saliva kill hiv"